27+ Henderson-Hasselbalch Equation Problems Pdf
This equation is very useful in calculating the pH of a solution. Calculate the pH of the solution that results when 400 mL of 0100 M NH 3 is.

Applications And Example Problems Using Henderson Hasselbalch Equation Analytical Chemistry Pharmaxchange Info
By definition does not dissociate completely and we can say and.

. 3 is to neglect x both in the parentheses in the numerator and in the denominator of eq. Buffers and the Henderson-Hasselbalch Equation Problems 11 - 20 Ten Buffer Examples Buffer Problems 1-10 Buffer. The Henderson-Hasselbalch equation fails to predict accurate values for the strong acids and strong bases because it assumes that the concentration of the acid and its conjugate base at.
Adding as little as 01. To begin convert the acid-dissociation constant into pKa. Is simply the logarithmic form ofthe Ka expression for the conjugate acid.
Approximate pH values obtained from the Henderson-Hasselbalch equation are compared with exact hydrogen ion concentrations and the percentage errors are displayed as. It can also tell you what the pH of the solution will be upon addition of strong acid or strong base. A Diluted with 200 mL of distilled.
Acid base problems - AP level Problems 1 - 10 Problem 1. 3 Use the Henderson-Hasselbalch Equation. Example 681 Exercise 681 Representing Buffer Solutions with Ladder Diagrams.
The Henderson-Hasselbalch equation can then be used to calculate the pH of this solution. The Henderson-Hasselbalch H-H equation provides a recipe for making a buffer of a given pH. PKa log Ka pKa log.
Systematic Solution to Buffer Problems. In this video lecture we solve several example problems in Henderson Hasselbalch equationsBiology Lectures is a research organization with the mission of. 1238 p H p K l o g H C O 3 003 P C O 2.
Note that is a weak acid. In this way we. PH 4752 log 000350 mol0060 L 00015 mol0060 L pH 4752 log 2333 pH 4752 0368 5120 Note the inclusion of.
View 5pdf from CM123 1P at Mapúa Institute of Technology. This form of the ionization or dissociation constant expression is called the Henderson-Hasselbalch equation. The Henderson-Hasselbalch equation is derived from this acid ionization constant.
To make our equation simple to use we now get rid of the negative log and so get the following equation 1238. The Henderson-Hasselbalch equation can give you more than just the pH of solutions. 2 Approximation to equation 3 An obvious way of deriving eq.

Write The Henderson Hasselbalch Equation For Acidic Buffer
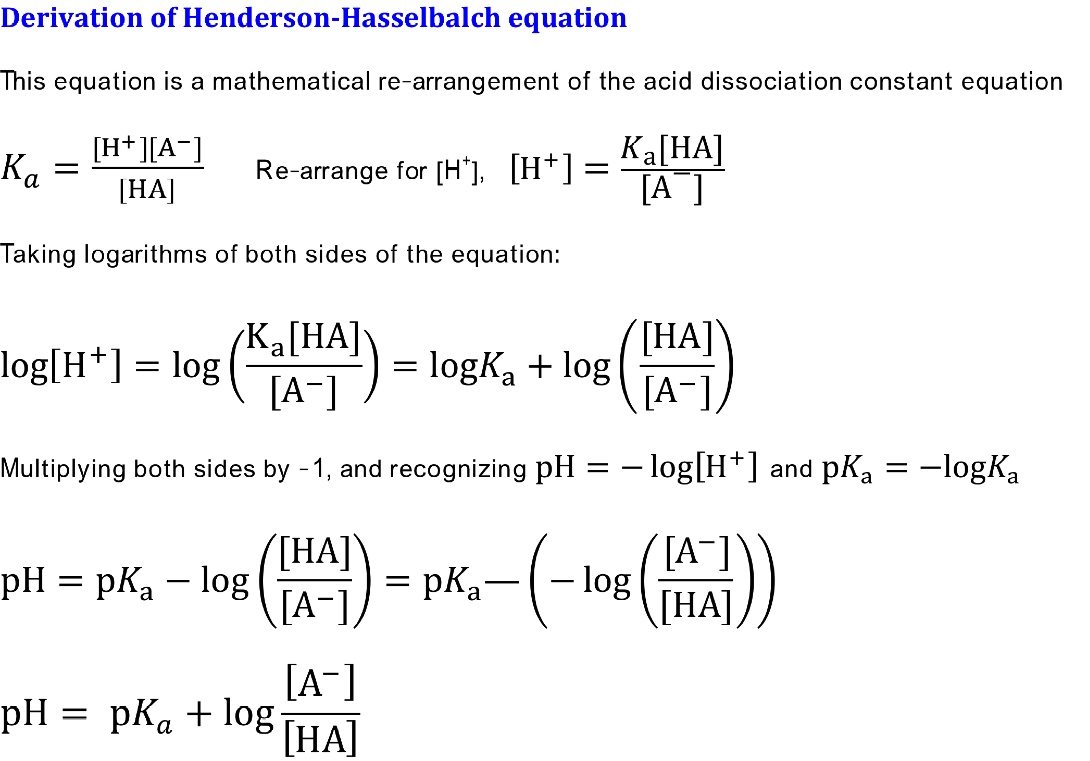
Derivation Of Henderson Hasselbalch Equation Secondary Science 4 All
Ppt The Henderson Hasselbalch Equation Powerpoint Presentation Free Download Id 4499608
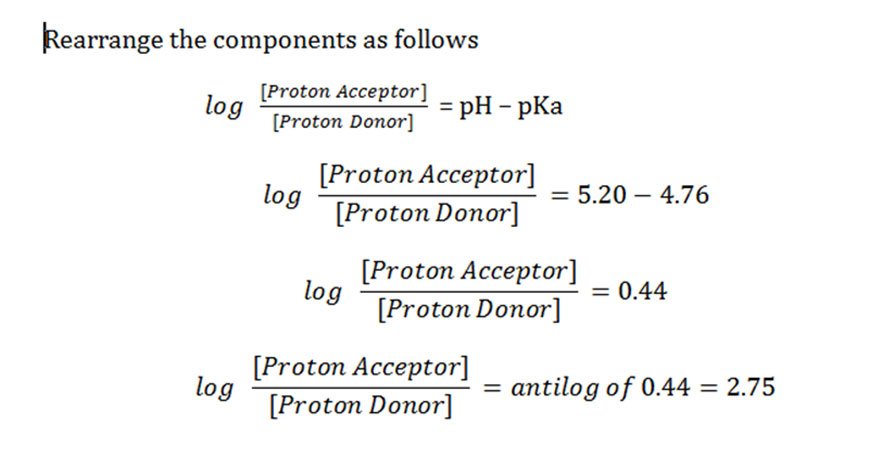
Solved Problems Henderson Hasselbalch Equation Ph Pka Easy Biology Class
Henderson Hasselbalch Equation Youtube

Henderson Hasselbalch Example Youtube

Henderson Hasselbalch Equation Questions Practice Questions Of Henderson Hasselbalch Equation With Answer Explanations

Pdf The Henderson Hasselbalch Equation A Three Dimensional Teaching Model
Henderson Hasselbalch Equation Practice Problems Medium
Applications Of Acids Bases

Pdf Using Activities To Correct The Henderson Hasselbalch Equation
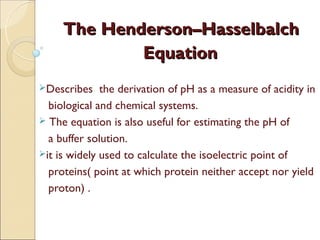
Henderson Hasselbalch Equation
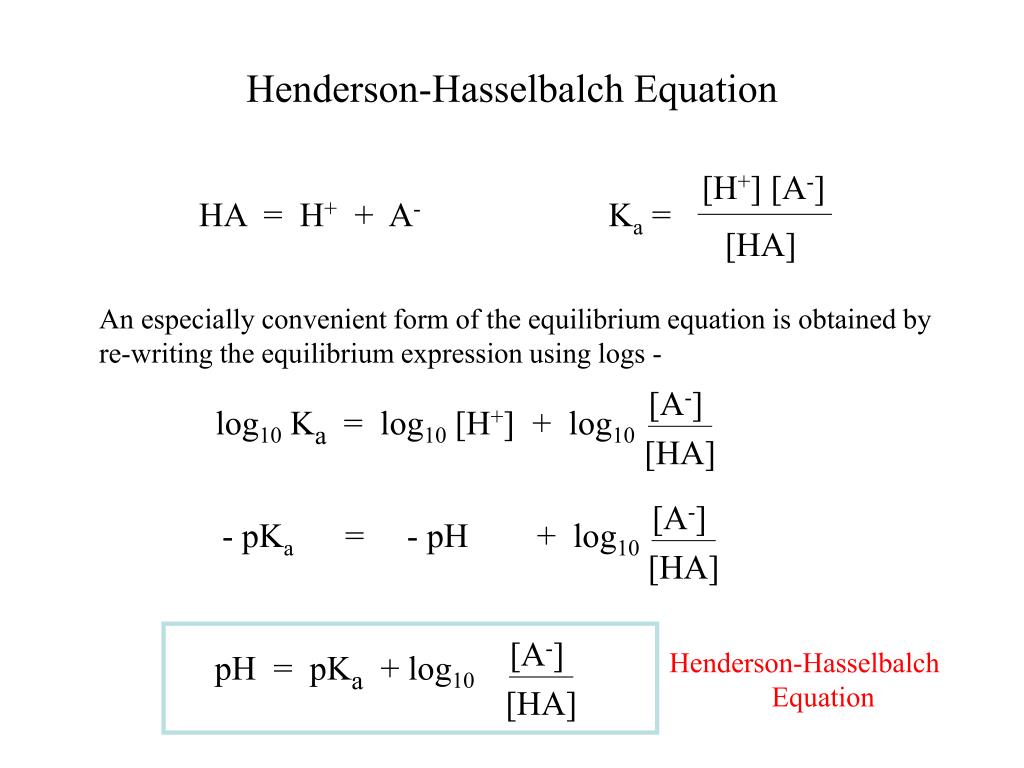
Ppt The Henderson Hasselbalch Equation Powerpoint Presentation Free Download Id 4499608

Buffered Solutions Henderson Hasselbalch Equation By Ricardo Elizondo

Henderson Hasselbalch Equation

Buffers And The Henderson Hasselbalch Equation Worksheet Exercises Chemistry Docsity
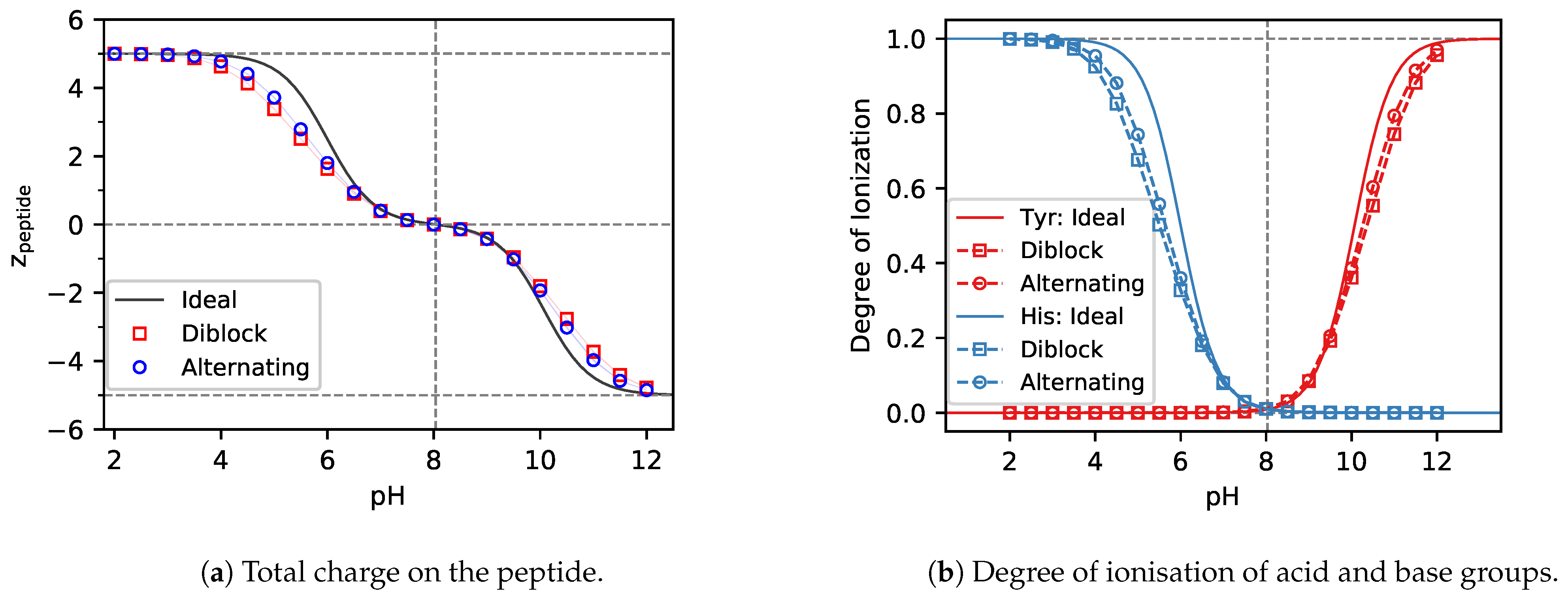
Polymers Free Full Text Role Of Pka In Charge Regulation And Conformation Of Various Peptide Sequences